Programs
Atrasentan
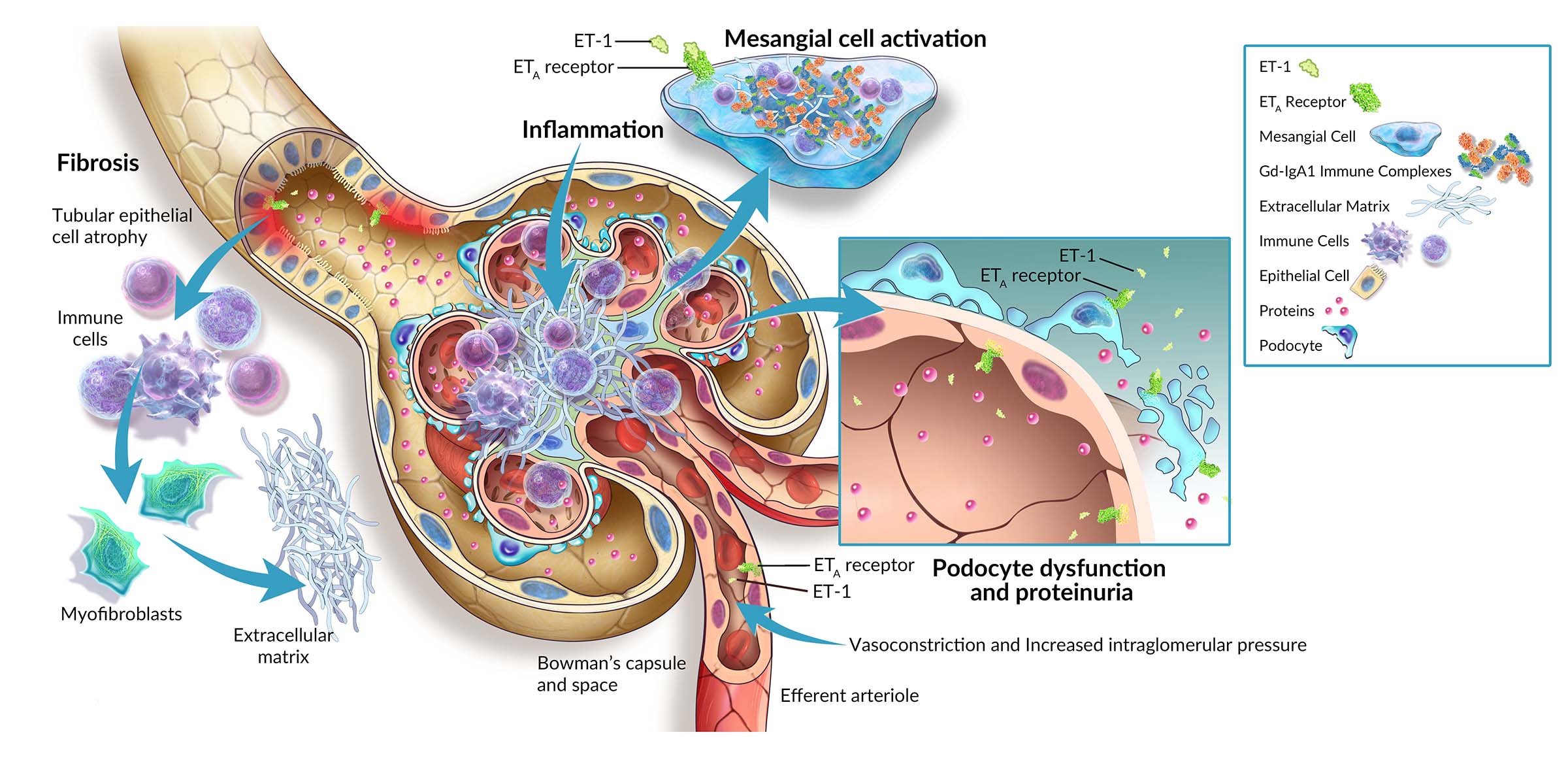
Atrasentan is a potent and selective endothelin A receptor antagonist with the potential to provide benefit in IgA nephropathy (IgAN) and other proteinuric glomerular diseases by reducing proteinuria.
Atrasentan in Action
Watch our video to learn more about atrasentan’s proteinuria-lowering mechanism of action as well as its anti-fibrotic and anti-inflammatory properties in proteinuric glomerular diseases.
We are currently conducting the phase 3 ALIGN trial of atrasentan for IgAN, phase 2 AFFINITY basket trial for proteinuric glomerular diseases, and phase 2 ASSIST crossover trial for individuals with IgAN on stable doses of a renin-angiotensin system inhibitor (RASi) and an SGLT2 inhibitor (SGLT2i). We identified IgAN as the lead indication for evaluation of atrasentan due to the role of endothelin activation and proteinuria in IgAN disease progression and high unmet need with the potential to submit an NDA seeking accelerated approval based on surrogate endpoints.
We in-licensed atrasentan from AbbVie in December 2019, which previously developed atrasentan for diabetic kidney disease (DKD) through multiple clinical trials, including a phase 3 SONAR trial, which evaluated atrasentan in over 5,000 DKD patients. In SONAR, atrasentan showed a statistically significant p-value of 0.029 on its primary endpoint of a composite of hard kidney outcomes (i.e., first occurrence of progression to end-stage renal disease or doubling of serum creatinine). The study also demonstrated statistically significant reductions in proteinuria as well as improvements in eGFR, both of which are measures of kidney function.
endothelin A receptor
Atrasentan is a potent and selective inhibitor of the endothelin A (ETA) receptor, which has the potential to provide benefit in multiple chronic kidney diseases by reducing proteinuria and having direct anti-inflammatory and anti-fibrotic effects to preserve kidney function.
Clinical trials
The ALIGN study, a phase 3, randomized, double-blind, placebo-controlled trial of atrasentan in patients with IgAN at risk of progressive loss of kidney function, is designed to evaluate change from baseline in proteinuria and eGFR in 320 patients with IgAN. Sustained proteinuria is the most widely studied and the strongest predictor for the rate of progression to ESKD in IgAN and may be accepted as a surrogate endpoint for regulatory approval. Sustained proteinuria is the most widely studied and the strongest predictor for the rate of progression to ESRD in IgAN and may be accepted as a surrogate endpoint for regulatory approval. This global study is being conducted in approximately 20 countries across four continents at approximately 160-170 investigative sites. For more information, visit ClinicalTrials.gov (NCT04573478).
The AFFINITY study, a phase 2 open-label basket trial of atrasentan in additional proteinuric glomerular diseases, is currently enrolling patients with FSGS. The four cohorts consist of patients with: IgAN with urine protein to creatinine ratio (UPCR) of 0.5 to less than 1.0 g/g, focal segmental glomerulosclerosis (FSGS), Alport syndrome and diabetic kidney disease (DKD) in combination with an SGLT2 inhibitor. For more information, visit the AFFINITY study website, visit ClinicalTrials.gov (NCT04573920).
The ASSIST study, a phase 2, double-blind, placebo-controlled crossover trial, is evaluating the safety and efficacy of atrasentan vs. placebo in subjects with IgAN on stable doses of a RASi and an SGLT2i. For more information, visit ClinicalTrials.gov (NCT05834738).
